Scientists Turn To Nature For Clues On Building Better Fuel Cells

New Research Center To Develop Effective Ways To Store And Use Chemical Energy
By Dick T. Co
Although hydrogen atoms are the lightest chemical element around, scientists are definitely not taking them lightly in their quest for alternative energy solutions.
Researchers at the Center for Molecular Electrocatalysis are dissecting nature's 3-billion-year-old photosynthetic machineries and reassembling the critical parts into new molecular systems that will effectively and affordably generate, store, and use clean energy.
Established in 2009, the Center for Molecular Electrocatalysis is one of the Department of Energy's 46 new Energy Frontier Research Centers. It received $22.5 million of funding over five years, the largest amount awarded for all of the EFRCs. The Center is based at the Pacific Northwest National Laboratory and comprises more than a dozen researchers from PNNL, the University of Washington, Pennsylvania State University, and the University of Wyoming.
All fuels used today, whether to generate electricity or to power your vehicle, ultimately came from photosynthesis. The oil, coal, and natural gas retrieved from the earth were once plant and animal material from hundreds of millions of years ago. As global energy demand is projected to grow at exponential rates this century, continual reliance on fossil fuels is not environmentally sustainable. The current release of the sequestered carbon back into the atmosphere in the form of carbon dioxide is simply too much too fast. Most would agree that greater use of non-fossil-fuel sources is needed to alleviate our dependence on foreign oil and curtail greenhouse gas emissions.
Many alternative energy sources today, such as solar and wind power, are intermittent. "We need a way to store that energy when the sun isn't shining and when the wind stops blowing," says Morris Bullock, the director of the Center. Fuels such as chemicals are far better at storing energy than batteries because they can pack a lot more energy into a given volume, a property known as high energy density. "So, converting electrical energy generated by solar and other power sources into chemicals could turn intermittent sources into reliable fuels."
Hydrogen is one attractive fuel option because it can be derived from water, an abundant and carbon-free source. However, it is very strenuous to convert water molecules into their constituent oxygen and hydrogen gases, a process known as "splitting water." Existing processes that split water cannot solve our energy problem because they either consume more energy than they produce or they use materials that are too expensive to be economical and scalable. New breakthroughs are required before we can turn water into a common clean energy source.
 |  |
The focus of the research at the Center is not on water splitting but on key reactions that move positive hydrogen ions, called protons, around to store energy in chemicals and other processes that produce electricity from chemicals. There are literally thousands of such reactions to explore, but luckily nature has done some work for us already. After nearly 3 billion years of evolution, nature has produced amazingly efficient molecular systems that use abundant materials to get the job done, and scientists are hoping to gain some insight from these systems.
Plants and photosynthetic microbes have perfected the process of capturing energy from the sun and converting water and carbon dioxide into energy-rich fuels. Although there are different flavors of photosynthesis in different species, the motif is usually similar. One part of the plant gathers the required energy by absorbing light. Another part uses that light energy to split water into oxygen gas, electrons, and protons.
Once the protons are extracted from water, some organisms then use enzymes called hydrogenases to efficiently combine two protons and two electrons to form hydrogen gas. The formation of hydrogen is the simplest fuel generation reaction.
Enzymes are a type of catalysts, substances that make chemical reactions proceed, over and over again, without being consumed by the reaction itself. They allow chemicals that ordinarily won't react with each other to react. "We are designing highly active catalysts for reactions that convert electrical energy into chemical bonds in fuels," explains Bullock.
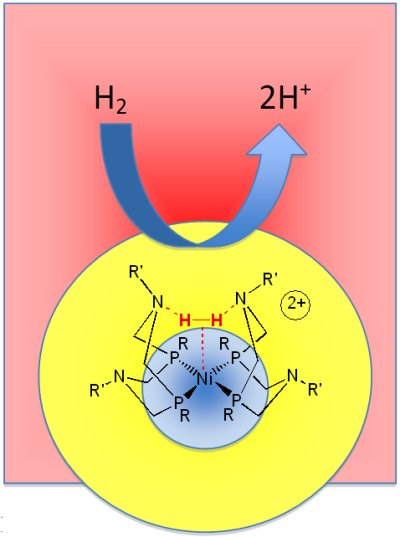
Over the years, scientists from around the world have learned that the hydrogenase enzymes use base metals like nickel or iron to hold on to the hydrogen and pendant nitrogens, or amines, to deliver and remove the protons. Bullock notes that the Center's aim is not to replicate the molecular structures found in nature but to build functional models using such metals and pendant amines.
An amine is a group of atoms containing a basic (alkaline) nitrogen, which has a natural affinity for protons. The pendants, also called proton relays, act as arms to help bind the hydrogen to the metal and shuttle protons between the metal and its surroundings— a molecular assembly line of sorts.
Researchers at the Center have already created prototype nickel and cobalt catalysts with pendant amines to relay the protons. These catalysts can produce hydrogen at rates close to those of natural catalysts. The new Center will help the scientists better understand, predict, and control the flow of protons in such complex catalytic reactions, making them not just fast, but efficient as well.
In addition to the production of hydrogen fuel, Bullock and his colleagues are also studying catalysts and reactions that convert chemical energy in bonds to electrical energy, such as those in fuel cells. Fuel cell technology is nothing new: NASA used it on the Gemini spacecraft nearly half a century ago. The problem is that the most effective fuel cells were, and still are, based on rare and expensive catalysts like platinum. Taking similar approaches to the design of the hydrogen-producing catalysts, Bullock and his team are developing new catalysts that will be much cheaper to manufacture than the present ones, making implementation on a global scale more feasible.
As if moving four protons and four electrons in hydrogen fuel cells weren't hard enough already, Bullock is also working on catalysts to be used in creating ammonia for fertilizer, a process that requires adding six protons and six electrons to diatomic nitrogen. Creating ammonia for fertilizer consumes one percent of the world's total energy supply. Improving the efficiency and lowering the cost of this reaction could have a huge and immediate impact on energy conservation and the improvement of the quality of life of millions.
"We hope to learn how to apply these principles to design a broader range of catalysts for energy applications," says Bullock. "The EFRC has allowed us to bring together a diverse group of researchers to attack this problem from different angles."
Dick Co is an alumnus of the 2009 Chemistry Communication Leadership Institute at the University of Washington. He is currently a research assistant professor of chemistry at Northwestern University and the Argonne-Northwestern Solar Energy Research (ANSER) Center, another EFRC.
Images
Top: Nature's way of converting sunlight into fuels while looking beautiful. Photo: Dick T. Co
Middle: A prototype catalyst comprising a nickel metal center and four pendant amines to be used in fuel cells. A diatomic hydrogen (in red) is seen coordinated to the catalyst. Image: Center for Molecular Electrocatalysis
Bottom: Morris Bullock (front right), Dan DuBois (front left), and their team of scientists at the Center for Molecular Electrocatalysis. Dan DuBois is the Deputy Director of the Center. Photo: Center for Molecular Electrocatalysis

|